The following letter (page 74) was sent by a British gastroenterologist to the commissioner of the Food and Drug Administration in the USA Dr.
KESSLER.
In the meantime the Food and Drug Administration dropped the Warning Box pertaining to gastric carcinoid tumors in animals. Nonetheless we feel obliged to publish
what Dr. WORMSLEY has written to an expert body for drug regulation, because of the severe allegations and possible implications for clinical medicine.
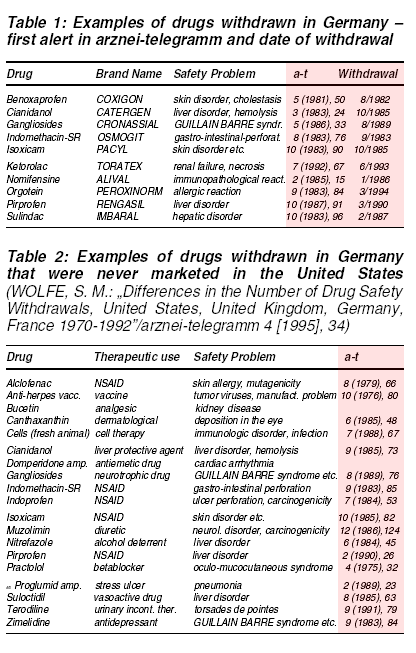
Reviews of recently suspended drug licences in central Europe and US show health regulatory bodies are rather reluctant to take action before an overwhelming
body of evidence has accumulated (tables 1 and 2).
It is our opinion, that a continuous evaluation of drugs is needed. In case of newly detected side effects of omeprazole government officials should be entitled to ask
for more and new safety data, especially for patients at risk - e.g.
- those with impaired redox defense mechanism,
- multiple organ failure,
- intracellular acidosis,
- subjects known as slow metabolisers,
- severe thiamine deficiency,
- otherwise severely compromised subjects like burn patients.
We believe, that we know the outcome of long term treating of patients with peptic ulcer with antisecretory agents in use since 1976.
Dr. WORMSLEY argues Ïhuman beings swim in a sea of carcinogensÙ while animal testing of a drug such as omeprazole happens in a largely carcinogen free
environment, and long term experience with proton pump inhibitors is lacking.
We invited the manufacturer of omeprazole twice (page 75) to comment on Dr. WORMSLEY's concerns. Astra Hässle (Sweden) felt not ready to do so, since they
have concerns about interfering in the pending issue.
* |
The induction of CYP IA1 activity1,2 in humans by omeprazole is observed not only in the intestine but throughout
the body.3 This induction is most likely mediated via activation of the Ah (Dioxin) receptors.4 Transcriptorial regulation of a
battery of human genes by omeprazole shows similarities and differences to known carcinogens such as dioxin and methylcholanthrene.5
Omeprazole is the only drug used worldwide in human therapy that is identified to have this unique property 6 - Red.
|
1 |
McDONELL, W. M. et al.: Gastroenterology 103 (1992), 1509
|
2 |
ROST, K. L. et al.: Clin. Pharm. Therapeutics 52 (1992), 170
|
3 |
McDONELL, W. M. et al.: Proc. North-South-Dialogue, July 1st, 1995, Innsbruck, in press
|
4 |
QUATTROCHI, L. C., TUKEY: Mol. Pharm. 43 (1993), 504
|
5 |
QUATTROCHI, L. C. et al.: Proc. North-South-Dialogue, ibid., in press
|
6 |
KLOTZ, U. et al.: Proc. North-South-Dialogue, ibid., in press
|
|

